
If an individual experiences anaphylaxis after being vaccinated with one type of COVID-19 vaccine, this does not preclude them from having another type following the additional precautions above. LatexĪll of the COVID-19 vaccines available for use within Australia (Vaxzevria (AstraZeneca), Comirnaty (Pfizer), Spikevax (Moderna) and Nuvaxovid (Novavax)) can both be administered to people with latex allergies following standard precautions, with a 15-minute post-vaccination observation period.

NB: Vaccination with Vaxzevria (AstraZeneca) or Nuvaxovid (Novavax) is contraindicated in people with documented anaphylaxis to Polysorbate 80.

If there is a history of confirmed or suspected allergy to Polysorbate 80 it is recommended that specialist advice be sought from an immunology/allergy/vaccination specialist regarding the safety of administering either vaccine. Polysorbate 80 is chemically related to Polyethylene Glycol and is an ingredient in both Vaxzevria (AstraZeneca) and Nuvaxovid (Novavax). NB: Vaccination with the Comirnaty (Pfizer) and Spikevax (Moderna) is contraindicated in people with documented anaphylaxis to PEG. It is recommended people with a history of confirmed or suspected allergy to PEG seek specialist advice from an immunology/allergy/vaccination specialist regarding the safety of vaccination. Whilst it is uncertain whether PEG contained in mRNA vaccines may trigger anaphylaxis, additional precautions are required prior to administration. It is also a commonly used ingredient of other medications, hand sanitisers, cosmetics, bathroom products and colonoscopy preparation products, routinely used within Australia. PEG is an ingredient contained in mRNA COVID-19 vaccines (Comirnaty (Pfizer) and Spikevax (Moderna)). SAEFVIC staff may direct the report to an immunisation specialist or alternatively to a vaccine allergy specialist within the VicSIS network (a network of specialist immunisation clinics in Victoria), as appropriate.Īllergy to components of COVID-19 vaccines Polyethylene Glycol (PEG)
Most of these cases (89%) occurred within 30 minutes of vaccination and 26% had a history of prior anaphylaxis.Ī confirmed vaccine allergy usually requires a specialist consultation with a vaccine allergy specialist, often with specific testing or a vaccine challenge under supervision.Īll COVID-19 immunisation hypersensitivity/allergic reactions should be reported to your state’s vaccine safety reporting service. Data from the US has shown that Spikevax (Moderna) has a rate of anaphylaxis with approximately 2.5 cases per million. Anaphylaxis following Comirnaty (Pfizer), whilst still extremely rare, occurs at a slightly higher rate of approximately 4.7 cases per million doses. Post-licensure surveillance of COVID-19 vaccines show anaphylaxis following administration of Vaxzevria (AstraZeneca) occurring at similar rates to routine vaccines. For further information on diagnosing hypersensitivity reactions and anaphylaxis please refer to Guidance for differentiating anaphylaxis and acute stress response for vaccine providers.
ALLERGIC REACTION TO MODERNA SKIN
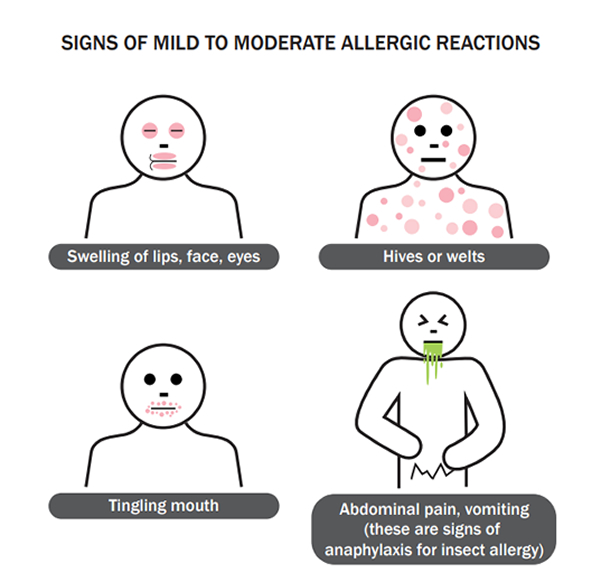
Suspected hypersensitivity reactions, particularly non-urticarial skin rashes following immunisation, are common, however true vaccine allergy, where a person is contraindicated from being immunised with the same vaccine in the future, is rare (in most studies reported as less than 1 case per million doses).
Hypersensitivity/allergic reactions following immunisation can be classified as:


 0 kommentar(er)
0 kommentar(er)
